Table of Contents
No one enjoys being sick. Bacteria, viruses, parasites — all are able to infect us and cause illness. Thankfully, our bodies are equipped with an immune system designed to protect us from these pathogens.
But our immune system doesn’t always win out. Infectious diseases have been responsible for the largest global burden of premature death and disability throughout for most of our evolutionary history, taking a backseat to chronic diseases only within the last 2–3 decades thanks to improvements in sanitation and medications like antibiotics and vaccines.
At a global level, infections are currently responsible for about 15% of all deaths, but most of this comes from developing nations where infectious diseases remain the leading cause of death. I’m not talking about complex viruses like HIV, either — I’m talking about respiratory and intestinal infections.
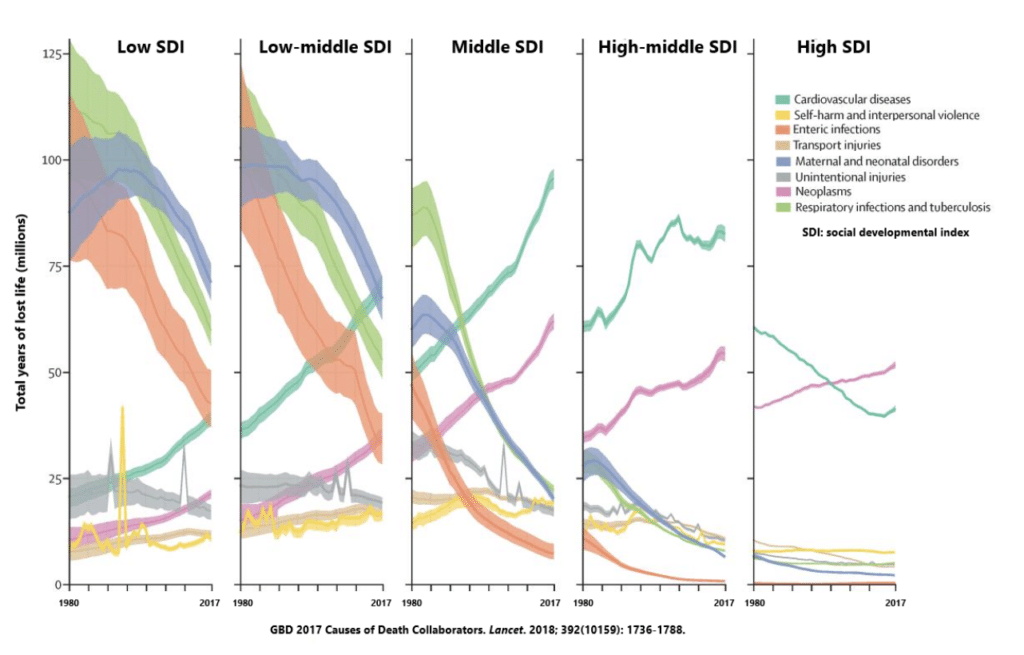
The sad truth is that developing nations are fighting a battle of starvation and poverty. It’s been known for some time that infectious risk and malnutrition are linked in a vicious cycle where malnutrition impairs immune defenses and increases the risk of becoming infected, which in turn can cause malnutrition.
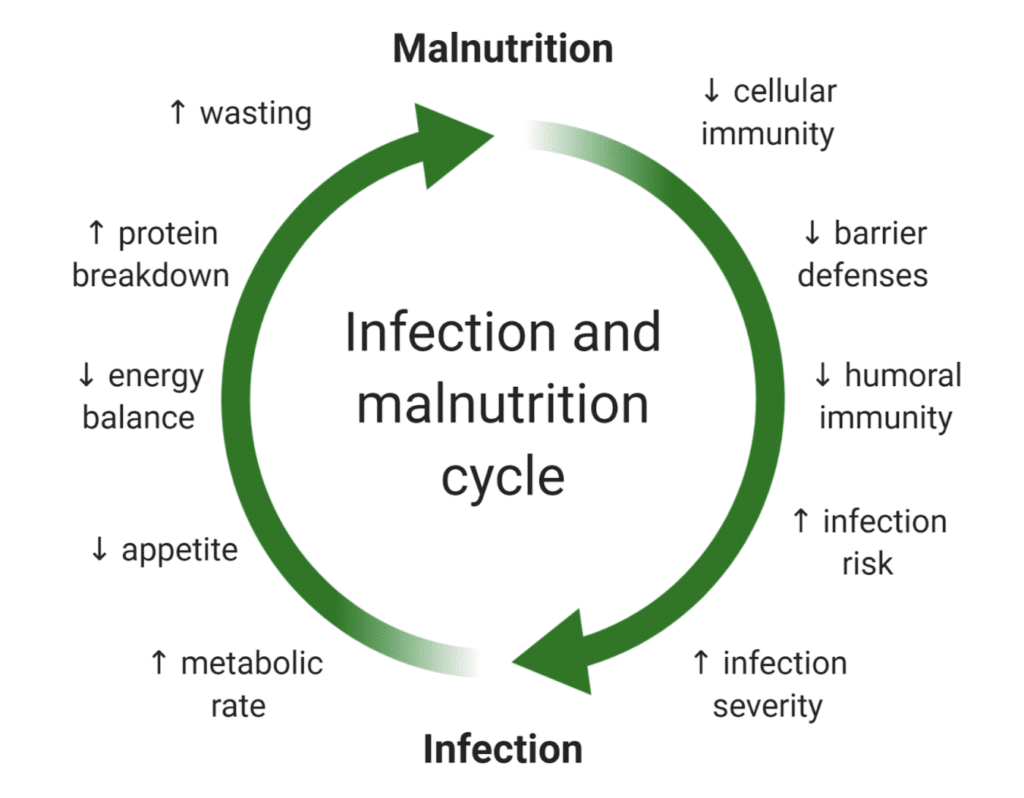
There aren’t many malnourished folks in developed countries like the US. Yet, respiratory infections are still the 6th leading cause of death and 7th leading cause of lost life due to premature death — that’s more than diabetes, stroke, or breast cancer. Why? How?
While a lot of infectious focus has been given to malnutrition, it turns out that being on the opposite side of the energy spectrum can really take a hammer to the immune system too.
Obesity is a big infectious risk
What may come as a surprise to you is that the link between obesity and infectious risk wasn’t heavily investigated until relatively recently. It all started around 2009 with the H1N1 flu pandemic — one that was estimated to claim the lives of 150,000 to 575,000 people.
Researchers from across the world were interested in figuring out what made some people more susceptible to contracting and dying from the virus.
- When researchers from Paris University in France meta-analyzed the data from 6 studies, they found that obesity was associated with an 8-fold greater risk of hospitalization and 2-fold greater risk of death.
- When researchers from China meta-analyzed 20 studies with over 25,000 flu cases, they found that obesity was associated with an 80% greater risk of dying from the flu, a 67% greater risk of having some form of a critical complication like respiratory distress syndrome, and a 2.5-fold greater risk of having some form of a severe complication that wasn’t life-threatening.
In light of the evidence surrounding the H1N1 flu, John Beigel from the National Institute of Allergy and Infectious Diseases sought to determine how obesity impacts the risk of flu-like illnesses in general. So, him and his team looked at over 3,000 adults that sought medical attention for flu-like symptoms and investigated how their symptom severity related to their body composition after taking into account things like age, sex, and chronic diseases.
Expectedly, Beigel found that being underweight was associated with a 3- to 5-fold greater chance of hospitalization for the flu and other respiratory tract infections. However, he also found a 3-fold greater risk with obesity and the flu and an 18-fold greater risk of morbid obesity and the flu. Regardless of body weight, those that had chronic diseases like diabetes and heart disease were at a 4- to 5-fold greater risk of hospitalization.
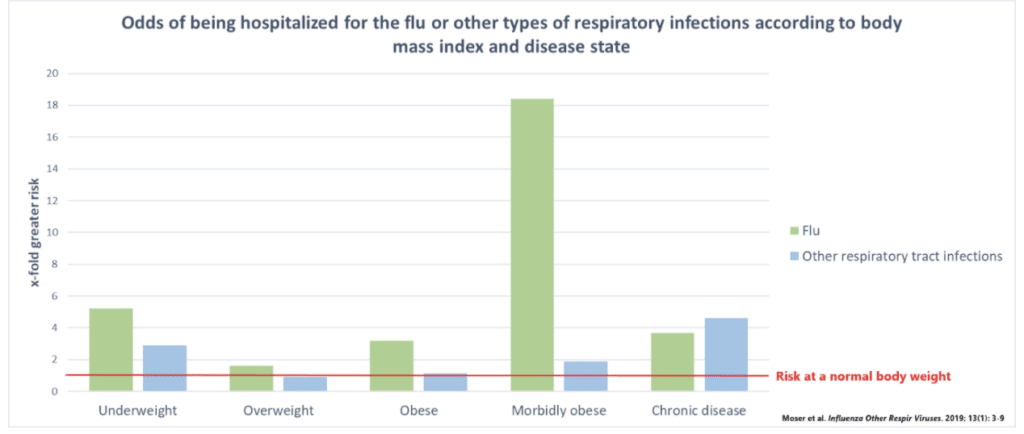
Basically, there’s a U-shaped curve when it comes to body weight and the risk of infection, where being either underweight or obese is a risk factor.
Of course, these are merely observations, meaning that we can’t say for certain that obesity causes worse immune function. And that’s precisely what Peter Eichacker and colleagues from the National Institutes of Health took issue with. To gain a better understanding of how body weight impacts survival when infected with bacteria or viruses, they conducted a systematic review and meta-analysis of 21 studies in mice and rats that became obese through either genetic or dietary means.
- Obesity caused premature death in 19 of the 21 studies.
- Compared to being normal weight, obesity reduced the chance of survival by 80%.
- The greater risk of dying was seen with both bacterial and viral infections.
One reason for the worse outcomes with obesity is simply that excess fat mass and state of low-grade inflammation impairs lung function and the ability to breathe, which has obvious implications for the severity of respiratory infections. However, the far more relevant reason behind worse infectious outcomes in those with obesity is that obesity impairs immune function.
This is why viruses are able to replicate, or shed, not only to a greater extent, but also for a longer period of time in those who are obese compared to those who are normal weight.
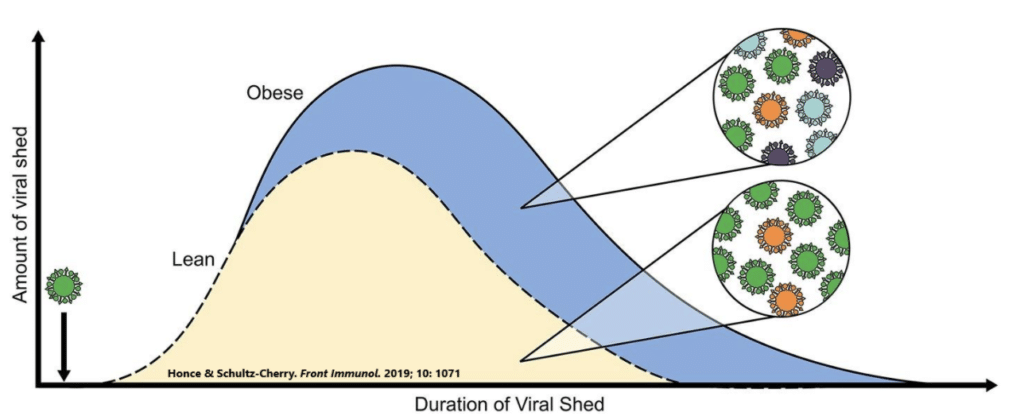
In fact, the obese body may be the perfect environment for viruses to establish a foothold. In a series of elegant studies conducted by Dr. Stacey Schultz-Cherry from St. Jude Children’s Research Hospital, diet-induced obese and normal-weight mice were inoculated with several types of flu viruses and followed as the infection took hold. Obesity not only led to greater mortality and severity of symptoms, but actually facilitated the mutation and replication of the viruses.
In other words, obesity promotes viral evolution and survival.
COVID-19
To bring our conversation to something incredibly relevant to today, I also want to touch on the relationship between obesity and COVID-19, which is the infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). I’m sure we are all familiar with it.
While this pandemic is only several months old, it’s becoming increasingly clear that obesity is one of the biggest risk factors for severe COVID-19 outcomes, particularly among younger individuals.
- For example, data from New York show that among those under 60, those with obesity were twice as likely to require hospitalization and 2- to 4-fold as likely to end up in intensive care for COVID-19 compared to those who were normal weight.
- Similarly, research out of France has found that, among those admitted to intensive care, being obese increased the risk of requiring invasive ventilation by over 7-fold.
- We also have several studies out of China reporting that overweight and obese patients were more likely to have severe COVID-19 infections and more likely to die from them.
Some researchers have proposed that fat tissue is a reservoir for COVID-19 and amplifies the inflammatory cytokine storm that causes severe respiratory complications in infected individuals. Collectively, this information has led other researchers to claim that any effective strategy for battling the current pandemic must involve diet and exercise that promote fat loss.
My goal with this article is to explain why and how obesity compromises immunity and creates such an opportunistic environment for infectious agents.
A quick overview of the immune system
Before jumping into a discussion on how obesity impacts immune function, we need to understand what the immune system is. I’m not going to go into extensive depth here — a brief overview is all that’s necessary.
At a fundamental level, the immune system is our body’s military force, constantly fighting to maintain our health against pathogens and infectious agents like bacteria, viruses, and parasites. Every bite of food, every breath, every cut or scrape — these all represent opportunities for microbes to invade our body and cause illness.
To fight off these invaders, our immune system has developed two military arms: (1) the innate immune system and (2) the adaptive immune system.
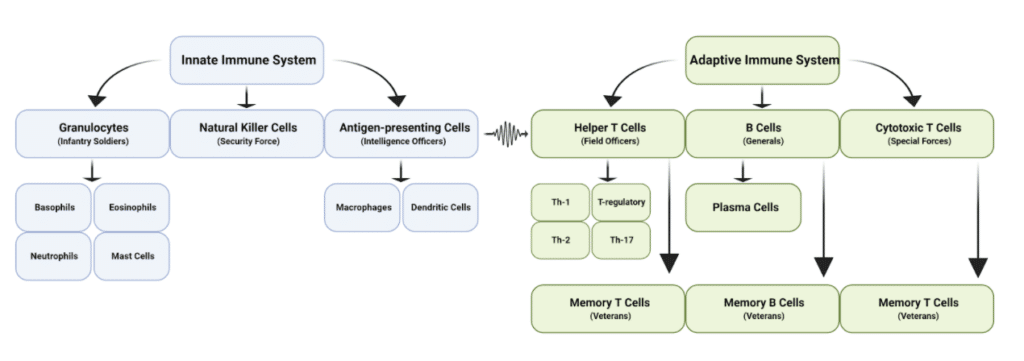
The innate immune system is our first line of protection against invaders and the bulk of our military force. It’s composed of white blood cells that patrol the body, attack anything they perceive as an enemy, and gather information about the enemy — all of which provides us with a broad, nonspecific defense against invading microbes.
- Our infantry unit is the granulocytes — the basophiles, eosinophils, neutrophils, and mast cells. This is the most abundant type of white blood cell in the body and the ones that battle our enemies in the trenches, using antimicrobial agents, enzymes, and reactive oxygen species to destroy them.
- Natural killer (NK) cells function as our security force, constantly in contact with cells throughout the body to ensure they aren’t compromised. If it notices any double-agents harboring viruses or traitors that are becoming tumorous, it releases cytotoxic agents that destroy our once-alley.
- The final cellular component of our innate immune system is the intelligence officers, which is represented by dendritic cells and macrophages. These cells specialize in capturing the enemy and extracting intel about their invasion before killing them, which is then provided to the adaptive immune system so that we can generate a specific immune response towards the enemy.
The adaptive immune system is our second line of protection against invaders and the commanders of our military force. These cells use the intel given to them by our intelligence officers to determine what type of attack is most effective against the enemy.
- Helper T-cells are the field officers of the immune system, being somewhat removed from combat but in direct communication with our innate immune forces. They use the information presented to them by our intelligence officers to dictate the movement and intensity of the innate immune attack against the enemy, as well as coordinate this attack plan with our military generals.
- Another type of T-cell that develops based on the type of information presented by our intelligence officers are cytotoxic T-cells. These guys are the special forces of our immune system, called in to eliminate very specific targets with minimal tissue damage, such as cancer cells, cells infected with viruses or bacteria, or cells that are damaged in other ways.
- The most far-removed immune cell from battle are the B-cells, which function as military generals that use the information given to them by our helper T-cell field generals to produce antibodies against the enemy, which function a lot like heat-seeking missiles — they seek out a specific type of pathogen and either destroy it or make it more susceptible to other immune cells.
- Lastly, we have the immune veterans, our memory T- and B-cells. These veterans form at the end of every battle and record information about the enemies faced in case they ever strike again. The next time our intelligence officers provide intel about the enemy that these veterans recognize, the retaliation is far more effective and lethal.
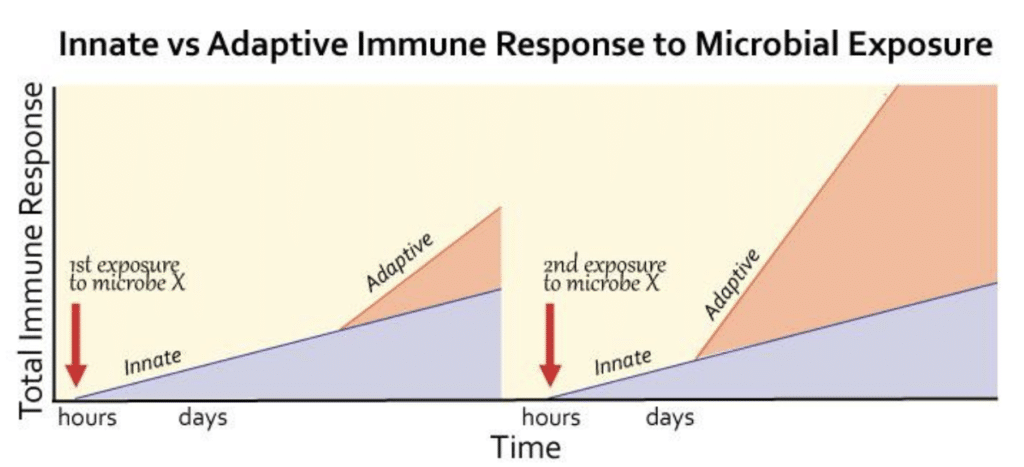
Ultimately, our immune response towards invading pathogens is a concert between the rapidly acting but relatively weak innate immune system and the slower but far more lethal adaptive immune system. The ability of the adaptive immune system to learn about the enemies it fights is a huge benefit for our survival, as it allows for a more rapid and more powerful response upon subsequent exposures to the same enemy.
Inflammation
So, where does inflammation come into play when we talk about the immune system? It’s a term that I’m sure most of you are familiar with. At the very least, you’ve probably heard it thrown around as the cause of numerous diseases. But, what is it?
Inflammation has always been a somewhat fuzzy and loosely defined concept. If you open up a pathology textbook, you’ll find a definition similar to the following:
“A response to infections and damaged tissues that bring cells and molecules of host defense from the circulation to the sites where they are needed in order to eliminate the offending agents.”
Of course, not everyone is happy with that definition. Dr. Irving Kushner from Case Western Reserve University, for example, argued that this definition was inadequate over 20 years ago since many conditions associated with inflammation don’t involve infections, damaged tissues, or apparent “offending agents.”
Instead, Dr. Kushner proposes that inflammation should be defined as:
“The innate immune response to harmful stimuli such as pathogens, injury, and metabolic stress.”
Immune cells release a variety of signaling molecules called cytokines. Those that increase the activity of the immune system are considered inflammatory and those that soften the immune response and switch it from an attack state to a repair state are considered anti-inflammatory.
So, when you hear the word inflammation, just think of an active immune system.
(Note: If you’re tired of always fighting a cold, taking sick days, and feeling worn out from chronic infections, then the easiest solution is to lose weight using the Fat Loss Blueprint. Grab it HERE.)
How obesity weighs down immunity
Now that we have a better understanding of our immune system and its military forces, we can appreciate the ways in which obesity affects it. Fundamentally, it comes down to the metabolic and hormonal changes that occur alongside carrying around too much fat.
Now, there are numerous metabolic and hormonal changes that occur with obesity, and they are all likely to play a role in one way or another. However, two changes stand out above all others:
- Chronic, low-grade inflammation
- Leptin resistance
Chronic, low-grade inflammation
At the top of our list is the inflammatory changes that often accompany obesity. In most people who are obese, lipid-overloaded fat cells stimulate innate immune defenses that secrete inflammatory signaling molecules into circulation, creating a state of chronic, low-grade inflammation throughout the body.
While tempting to believe that being a state of heightened immune activity would improve our defense against infections, it actually has the opposite effect. Much like a financial budget for a military, our body has limits on its ability to create and properly train immune cells, leading to a deterioration of immune function over time, something called immunosenescence.
- Infantry soldiers become weaker and move more slowly.
- Our security force isn’t as good at identifying and killing infected cells.
- Intelligence officers are worse at obtaining intel about the enemy.
- More field officers are retired and incapable of responding to an attack.
- The special forces lose much of their lethality.
- Our generals have difficulty targeting novel enemies that we haven’t faced previously.

Although immunosenescence occurs naturally as we age, this isn’t due to an intrinsic property of aging. Rather, it’s explained by an imbalance between inflammatory and anti-inflammatory signaling that results in a state of chronic, low-grade inflammation some researchers have termed “inflammaging.”
That same chronic, low-grade inflammation that causes immunosenescence is present in most people who are obese too.
The similarities between immune function in obesity and aging are ultimately what led Monica De la Fuente from the University of Madrid to propose that obesity is a model of premature immunosenescence. She later went on to support this proposition by showing that young obese mice have similarly impaired immune function as normal-weight more than twice their age.
If De la Fuente’s findings translate to humans, it would be equivalent to an obese 21 year-old having the immune system of a normal-weight 55 year-old!
And preliminary data in humans suggests this to be the case — there are few differences in the immune function of younger and older obese adults, suggesting that obesity does indeed cause premature immunosenescence. Moreover, incubating healthy immune cells with the plasma of obese adults causes them to dysfunction and display characteristics of immunosenescence.
To add insult to injury, immunosenescence and chronic, low-grade inflammation exist in a vicious cycle with one another. During immunosenescence, the adaptive immune system takes the biggest hit, which serves only to cause a compensatory increase in the innate inflammatory response. In other words, the failing adaptive immune system causes more chronic, low-grade inflammation in the body that caused it to fail in the first place.

Aside from promoting immunosenescence, chronic, low-grade inflammation may lead to the development of tolerance against inflammatory signals that would alert the immune system to an infection. Basically, it takes a stronger initial inflammatory response to infection to get the attention of immune cells, meaning there is a delay in the containment of pathogens that invade the body, which gives them a larger window of opportunity to spread or do damage.
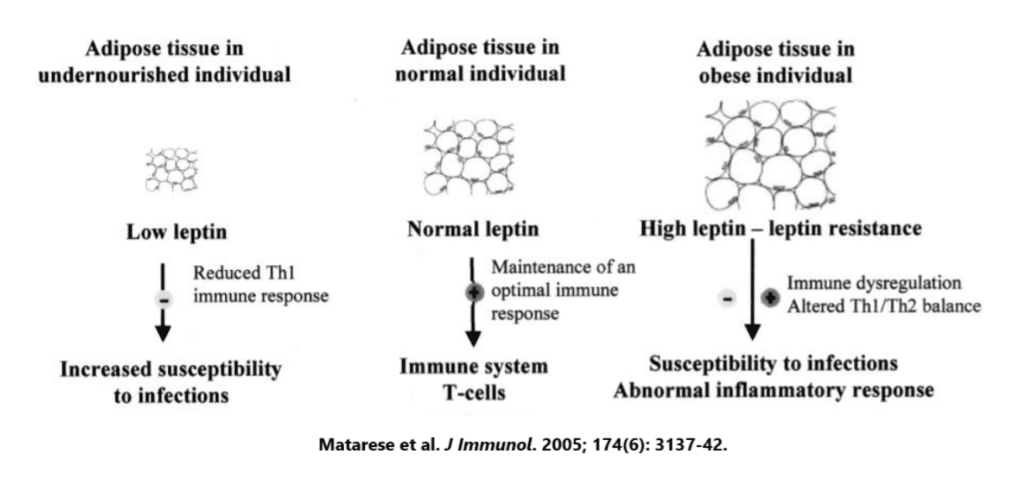
Leptin resistance
The second primary way that obesity reduces immune function is through promoting a state of leptin resistance. For the longest time, our fat cells were thought of as just a reservoir of energy for use at later times, and many people today still hold this view — that fat cells store excess energy from food, nothing more, nothing less.
It wasn’t until 1994 that researchers found definitive proof that fat cells were so much more than a place of energy storage — it was in 1994 that Jeffrey Friedman of Rockefeller University discovered leptin, a hormone secreted by fat cells that plays a central role in appetite regulation through reducing hunger and increasing satiety.
While best-known for its role in appetite regulation and the control of body weight, leptin also has diverse effects on the immune system. Nearly every immune cell has leptin receptors and leptin binding increases immune cell survival and ability to fight off invading pathogens — leptin is basically a pro-inflammatory molecule.
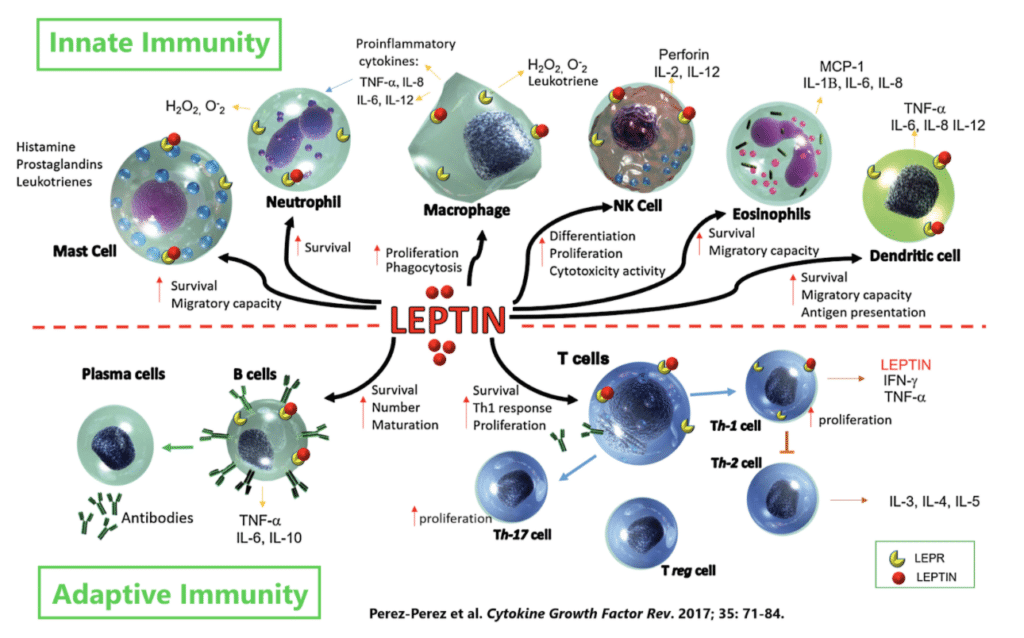
The amount of leptin we have circulating throughout the body is directly proportional to the amount of fat mass we are carrying around, meaning that the more obese you are, the more leptin you have. However, this doesn’t mean that there is greater leptin signaling in the obese body. Quite the contrary, actually.
Obesity is associated with a state of cellular leptin resistance.
This is why injecting leptin into folks with obesity causes little to no weight loss — the chronically elevated leptin levels caused their body to down-regulate the expression of leptin receptors, so there wasn’t any way for the increased leptin levels to have an effect.
We see the same type of resistance in immune cells. When mice are fed a fattening diet that has them become obese, their T-cell field officers and natural killer cell security forces don’t function properly due to a reduction of leptin signaling. In this way, obesity mimics the immunocompromised state seen with malnourishment.
Key points
The risk and severity of infections exist on a U-shaped curve with body weight, where being malnourished and overnourished are risk factors. Obesity increases not just the risk of severe complications when you become sick, but also the risk of dying.
To be immunocompromised is to have an immune system incapable of working at full capacity. You’ll get sick more often, stay sick longer, and be more vulnerable to different types of infections. Given everything we have discussed so far, it’s fair to say that if you’re obese, you’re immunocompromised.
Virtually every aspect of innate and adaptive immunity is impaired with obesity:
- Because obesity often coexists with a state of chronic, low-grade inflammation, infected cells are slower to release “help” signals that draw attention to the infection.
- As a result of delayed “help” signals, the innate immune system is less capable of containing the infection before it spreads, meaning more cells become infected.
- The delayed innate immune response is compounded by impaired functionality of innate immune cells like NK cells, macrophages, and dendritic cells.
- Impaired functionality of macrophages and dendritic cells means that T-cells don’t receive enemy intel in a timely manner, delaying the adaptive immune attack.
- When the adaptive immune system finally gets going, the helper T-cells are excessively inflammatory, the cytotoxic T-cells are inefficient, and the B-cells do not produce as many antibodies.
- If the infection is overcome, less memory T- and B-cells are formed, meaning that there is increased susceptibility to future infections by this specific enemy, and there is an impaired ability to cleanup damaged cells and repair any damaged tissue.

Want the Fat Loss Blueprint for Optimal Health and Sustainable Fat Loss?
If you’re serious about losing fat in the most efficient and healthiest way possible, then join our online program, where we’ll show you how to make the dieting process second nature, lose fat, and keep the fat off in the long-run.
In this program, we’ll take you through 18 of the most powerful science-backed strategies in existence for regaining and retaining that youthful body. We cover everything from fat loss fundamentals like getting enough protein to optimizing sleep and stress reduction to using supplements that actually work — all in the name of fat loss.
So if you’ve been struggling with your weight or want to push the boundaries of leanness, then you simply MUST join our Fat Loss Blueprint HERE.)



