 Most people know melatonin as the “sleep hormone.” And it is certainly that.
Most people know melatonin as the “sleep hormone.” And it is certainly that.
But most people have absolutely no idea that melatonin is holding another secret, that is, believe it or not, probably even more important than its role in sleep.
Melatonin is a powerful “Energy Hormone”!
No, melatonin is not a stimulant that acts to create an instant energy boost like caffeine or other stimulants.
It works to boost your energy levels in a much more important and powerful way that is less about an instant (but very short-lived) energy boost and more about building lasting, long-term high energy levels.
How does it work to do this?
 Well, of course, melatonin is vital for sleep quality — so even just sleep quality is obviously massively important to energy levels. But beyond that, melatonin has been hiding a secret from us that few people (even health experts) are aware of…
Well, of course, melatonin is vital for sleep quality — so even just sleep quality is obviously massively important to energy levels. But beyond that, melatonin has been hiding a secret from us that few people (even health experts) are aware of…
Melatonin is perhaps the single most potent protector of your mitochondrial health.
Of course, mitochondria are our cellular energy generators — those little batteries that supply virtually all the energy to the trillions of cells in our body.
It turns out that melatonin is absolutely vital for mitochondrial health.
So, here’s a frame shift for you to wrap your head around this: The most powerful antioxidant in existence at the cellular/mitochondrial level, is not vitamin C or vitamin E or acai or goji berries — it’s DEEP SLEEP!
Of course, sleep is not an “antioxidant” in the sense that you consume it in a pill and it gets absorbed into your body and acts as an antioxidant. It’s actually something hundreds of times more powerful than that.
There are actually two things going on that make sleep such a potent antioxidant:
- Melatonin is the most powerful mitochondrial antioxidant. Melatonin — the hormone our brain secretes to trigger sleep when it gets dark — is an incredibly potent and very unique kind of antioxidant. Importantly, unlike vitamin A, C, E and virtually all other antioxidants, melatonin is able to get into the mitochondria, where protecting cells from oxidative damage (free radicals) actually matters. (Note: Virtually all studies on vitamin A, C, and E supplements have failed to show benefits on aging and disease prevention,[48] and researchers now suspect this is why – because these antioxidants can’t get to the part of the cell where it really matters, the mitochondria.)
- Melatonin regulates and amplifies autophagy and mitophagy.[1][2] Autophagy is the process where cells chemically digest and recycle broken down and dysfunctional cell parts and then rebuild new healthy cell parts. Mitophagy is the same basic process, but specifically localized in the mitochondria. These processes have now been recognized as being absolutely critical to the prevention of numerous diseases (including protecting against cancer) and even the aging process itself. In addition, without mitophagy functioning optimally, you are functioning TODAY on YESTERDAY’S broken down and dysfunctional mitochondria. Over time, that means less energy. So, the process of mitophagy is absolutely vital for optimal cellular energy production.
- Melatonin builds the almighty internal antioxidant defense system. Even more important than any antioxidant we might consume in food or in a supplement (and even the direct antioxidant effects of melatonin) is our cells’ own internal antioxidant defense system. And it turns out that every night while you sleep, this cellular antioxidant defense system regenerates and replenishes its supply of antioxidants for the coming day.[3][4][5] guess what happens when you don’t sleep enough, or you sleep poorly? Of course, your mitochondria are chronically operating with low supplies of internal antioxidants, so they are chronically getting damaged. And when they’re chronically being damaged, they’re going to shut down energy production and focus all their energy into Defense Mode to protect themselves against threats.
Melatonin is essentially the mitochondrial antioxidant, and ancient one at that.
“Melatonin may be considered as a mitochondrial antioxidant because it not only directly scavenges and indirectly neutralizes free radicals, but it also reduces radical generation in mitochondria, via a phenomenon known as radical avoidance. To achieve radical avoidance, melatonin accelerates the electron flow through the [electron transport chain] and slightly activates the [mitochondrial permeability transition pore]. This may explain why melatonin is more protective of mitochondria against oxidative stress than other antioxidants. Studies have documented that melatonin restores the mitochondrial functions in aged animals and in animals with different pathological conditions.”[6]
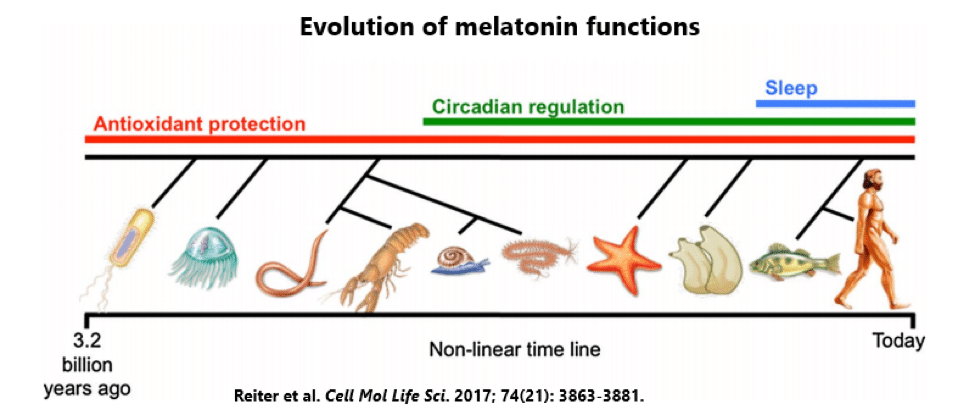
Melatonin is quite possibly nature’s most versatile biological signal. It has been found in all major types of organisms — not just animals, but also plants, algae, fungi, and even bacteria.[7] In fact, melatonin is believed to have appeared over 3 billion years ago in photosynthetic bacteria, where it protected against oxidative stress.[8]
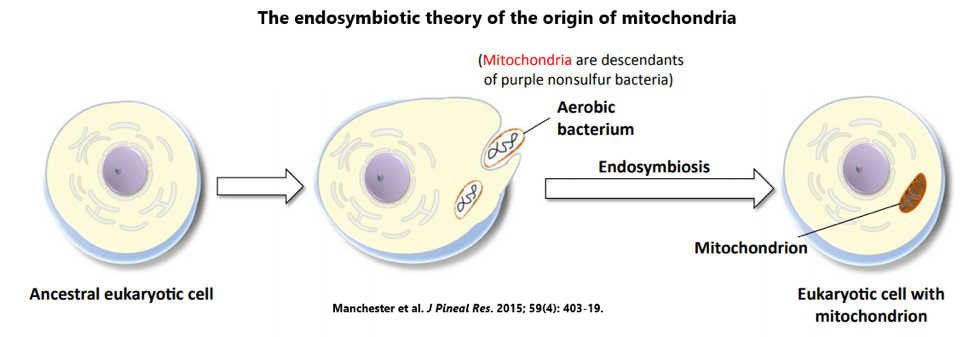
Yep, the so-called sleepy hormone started as an antioxidant, with its role in sleep and circadian regulation not appearing until much, much later.
What’s really amazing is that the purple nonsulfur bacteria that originally harnessed the power of melatonin are the same bacteria believed to have evolved into mitochondria.[9]
You would think that our bodies would have developed better antioxidant systems over the past 3 billion years, but that doesn’t seem to be the case.
Melatonin is arguably the most potent antioxidant in our body.
Unlike many other antioxidants such as vitamin C and glutathione, which can only neutralize one free radical at a time, melatonin has the ability to neutralize multiple free radicals at once and works synergistically with other antioxidants when combined. [10] [11] In fact, when one melatonin is lost to a free radical, three other antioxidants emerge to take its place.[12]
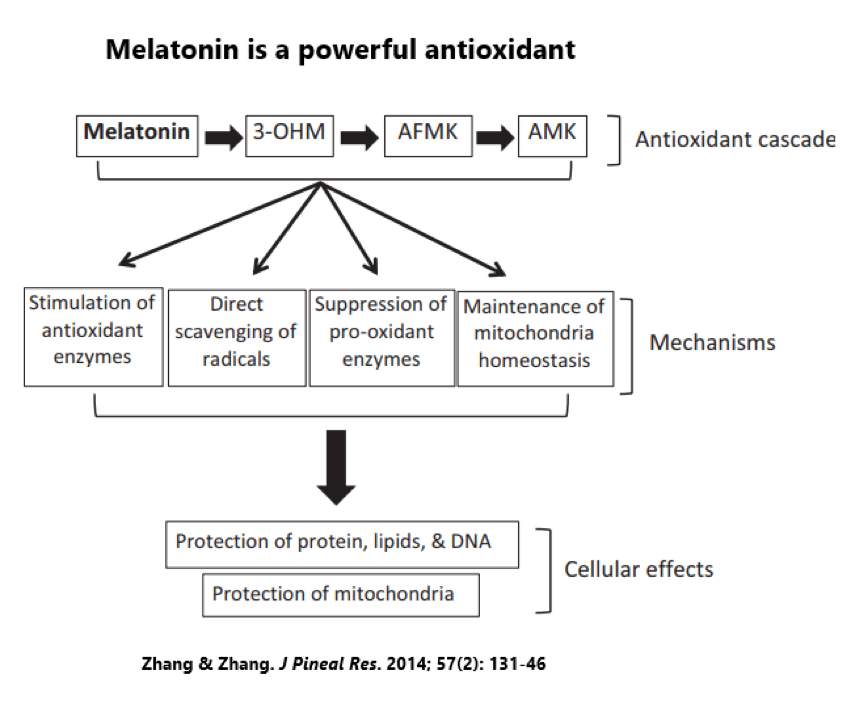
Melatonin and its derivatives work to stave off oxidants in several ways: (1) direct scavenging of radicals, (2) stimulation of antioxidant enzymes, and (3) suppression of pro-oxidant enzymes.[13]
The direct scavenging ability of melatonin is one of the most diverse within our bodies — melatonin can easily pass through cell membranes and reach every corner of our body, where it exerts an antioxidant ability twice as powerful as vitamin E and four times more powerful than glutathione or vitamin C.[14] Melatonin also motivates the worker-bees that create other antioxidants in the body, such as glutathione peroxidase, superoxide dismutases, and catalase.[15]
The antioxidant function of melatonin is absolutely vital for mitochondrial function, protecting our cellular powerhouses from the highly toxic free radicals they make as a byproduct of energy production.[16] Other antioxidants help out, but melatonin towers over all others. Even before mitochondria were mitochondria, the bacteria preceding them were using melatonin to protect themselves.[17]
There are a variety of synthetically altered antioxidants marketed as “mitochondrial targeted”, such as MitoE and MitoQ, which have vitamin E and coenzyme Q10, respectively, bound to a molecule that increases their ability to enter the mitochondria. And they work… well. These synthetically produced antioxidants accumulate in concentrations up to 500-fold greater than natural vitamin E and coenzyme Q10 counterparts.[18]
Here’s the kicker: Despite these high concentrations, when compared to similar amounts of melatonin that can occur under natural circumstances, melatonin is as good as or better than the fabricated antioxidants in protecting against mitochondrial oxidative stress![19] These findings and others have led some researchers to call melatonin the true mitochondrial-targeted antioxidant.[20] [21]
The Problem: An Epidemic of Low Melatonin Levels
Here’s the big problem: We now live in a world that is almost perfectly designed to suppress the secretion of melatonin by our brain each night.
The release of melatonin depends entirely on the presence of darkness, or more accurately, it depends on specific wavelengths of light (specifically blue and green colored light) not entering our eyes.
Humans are designed for an outdoor existence in tune with the rise and fall of the sun.
We’re not designed to be indoors almost all day, and then to be staring at all sorts of artificial light sources after the sun sets.
Evolutionarily speaking, humans had plenty of melatonin secreted every night. When the sun went down, their brains pumped out plenty of melatonin. But in the modern world, we have all kinds of artificial light (from indoor lighting, cell phones, TVs, and computers) blaring into our eyes each night after the sun goes down.
What does that do? It suppresses melatonin!
As long as you have blue light entering your eyes, it’s sending a “daytime signal” to your brain and that will suppress melatonin. Simply put: Virtually all of us in modern society are suffering from one degree or another of circadian rhythm disruption.
The visible spectrum of light ranges from about 380 to 800 nanometers (nm), with the low end full of purples and blues, and the high end full of reds and oranges. It is the blue and green wavelengths (about 380 to 550 nm) that mess with our biological clocks.[23] [24]
When blue and green light enter our eye, they signal to the brain that it is daytime by shutting down melatonin production.
Exposure to normal room lighting before bed reduces melatonin production during this time by an average of 71%![25]
So each night you are being exposed to typical room lighting and various electronic devices (phone, computer, TV etc.), you have dramatically less melatonin getting into your mitochondria than you should have – so you are directly contributing to the accumulated damage and dysfunction of your cellular energy generators (your mitochondria).
The modern world is polluted with artificial light at night that scatters through the skies and creates one of the most pervasive environmental alterations — artificial skyglow. About 83% of the world’s population and more than 99% of the U.S. and Europe live under light-polluted skies, with more than a third of humanity unable to see our galaxy and the stars it contains.[22]

The constant barrage of light has its uses for productivity, safety, and comfort, but it’s also a huge health concern given how it affects our sleep and circadian rhythms.
It needn’t just be regular lighting, either — playing on your phone, working on your laptop, and watching TV all have similar effects due to the blue-rich light they put out. It should therefore be no surprise that nighttime exposure to electronics is associated with worse sleep quality.[26] [27] [28] In fact, children who use media devices before bed are 1.5-fold more like to have worse sleep quality, 2.5-fold more likely to sleep less, and 2.7-fold more likely to be excessively tired during the daytime.[29]
In adults, living in areas with more light at night was associated with going to bed later, getting less sleep, having poor sleep quality, being excessively fatigued and sleepy during the day, and having circadian rhythm disorders.[30] Elderly folk living in areas with more artificial light at night are more likely to take prescription sleep drugs![31]
Circadian Disruption → Melatonin Suppression → Mitochondrial Dysfunction → Fatigue (and Disease)
The implications of disrupted melatonin secretion should be obvious given its role in maintaining mitochondrial function and squashing oxidative stress.
We used to have plenty of melatonin secreted every night when the sun went down, but today, all the light pollution is suppressing melatonin production — meaning that you are chronically increasing mitochondrial damage and indirectly accelerating aging and predisposing yourself to disease.[32] [33]
In other words, your mitochondria are chronically operating with low supplies of internal antioxidants, leading them to be chronically damaged, leading them to shut down energy production and enter into defense mode to protect themselves against oxidative stress.
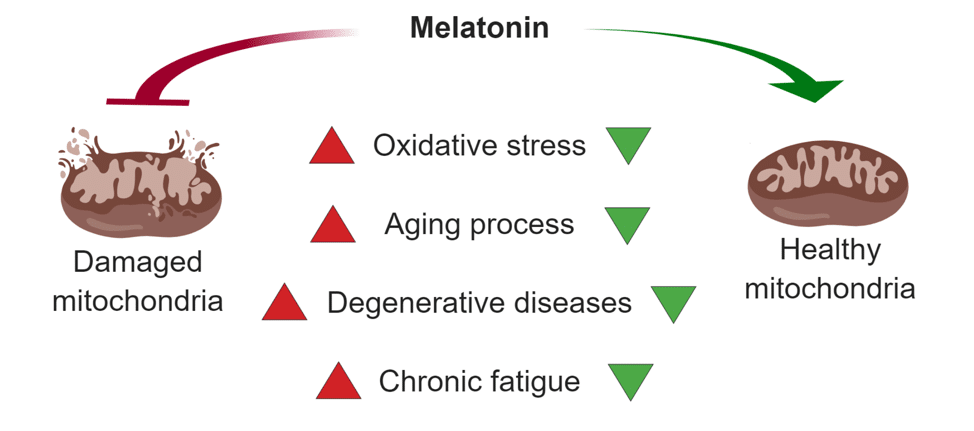
There are a number of impressive – and little known – studies showing how vital it is for mitochondrial health that you produce LOTS of melatonin each night while you sleep. The melatonin we release that prepares us for sleep nourishes the mitochondria in numerous ways including:
- Acts to prevent free radical damage directly in the actual mitochondria.[34][35][36][37] (which is very unique to melatonin since virtually all other “antioxidants” cannot do this)
- Regulates of mitochondrial bioenergetic function and maintains respiratory complex activities, electron transport chain, and ATP production in mitochondria.[38][39][40]
- Acts as a neuroprotectant in the brain, preventing the kind of oxidative stress/nitrosative stress-induced mitochondrial dysfunction seen in experimental models of Parkinson’s, Alzheimer’s, and Huntington’s disease [41]
- Slows aging [42][43]
And here’s the big problem: When you have poor circadian rhythm habits, you have less melatonin in your body that can get to the mitochondria.
Too much blue light at night (and blunted circadian rhythm in general, from poor circadian rhythm habits) greatly inhibits melatonin release, which directly leads to grossly negative impacts upon mitochondrial dysfunction, causing mitochondrial fragility and die-off.
Over time, what that means is that mitochondria will accumulate more damage, will not function as well, and you will not rebuild new healthy mitochondria nearly as well. You’ll be slowly accumulating more and more damaged and defective mitochondria, and inevitably, you will end up fatigued.
If we lose our mitochondria – or accumulate lots of weak and damaged mitochondria — we lose our health, energy, immunity, strength, and vitality as well. We age faster, we are far more susceptible to disease, and we end up chronically fatigued.
Is it starting to make sense why you’re feeling so tired?
If the mitochondria need to regenerate each night in order to manufacture the ATP that gives you energy, then without good circadian rhythm habits and lots of melatonin being pumped out of your brain each night while you sleep, you’ll slowly be sapping away your body’s ability to produce energy at the cellular level day after day.
You’ll be slowly accumulating more and more damaged and defective mitochondria, and inevitably, lose your health, energy, immunity, strength, and vitality. You’ll age faster, be far more susceptible to disease, and end up chronically fatigued.
Bottom line: Producing ample amounts of melatonin each night for an ample amount of time is vital for mitochondrial health. And mitochondrial health, in turn, is the single most important factor in longevity, the prevention of most chronic diseases (including cancer), brain health, and of course, your ENERGY levels.
If you want to know the ultimate science-backed blueprint for optimizing your circadian rhythm — so you pump out LOTS of melatonin each night, sleep deeper than you have since you were a kid and have more energy than you’ve had in decades — then I strongly encourage you to grab our Sleep & Circadian Rhythm Optimization Protocol HERE.
References
[1] Jenwitheesuk, A., et. al., (2014) Melatonin Regulates Aging and Neurodegeneration through Energy Metabolism, Epigenetics, Autophagy and Circadian Rhythm Pathways Int J Mol Sci. 2014 Sep; 15(9): 16848–16884. Published online 2014 Sep 22. doi: 10.3390/ijms1
[2] Coto-Montes, A., et al., Role of melatonin in the regulation of autophagy and mitophagy: A review https://doi.org/10.1016/j.mce.2012.04.009
[3] D’Almeida V., et. al. (1998) Sleep deprivation induces brain region-specific decreases in glutathione levels.
[4] Inoué S., et. al. (1995) Sleep as neuronal detoxification and restitution
[5] Everson CA., et. al. (2005) Antioxidant defense responses to sleep loss and sleep recovery
[6]Tan, D.-X. et al. Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin’s primary function and evolution in eukaryotes. J. Pineal Res. 54, 127–138 (2013).
[7] Pandi-Perumal, S. R. et al. Melatonin: Nature’s most versatile biological signal? FEBS J. 273, 2813–2838 (2006).
[8] Manchester, L. C. et al. Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 59, 403–419 (2015).
[9] Tan, D.-X. et al. Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin’s primary function and evolution in eukaryotes. J. Pineal Res. 54, 127–138 (2013).
[10] Zhang, H.-M. & Zhang, Y. Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 57, 131–146 (2014).
[11] Tan, D.-X. et al. Mechanistic and comparative studies of melatonin and classic antioxidants in terms of their interactions with the ABTS cation radical. J. Pineal Res. 34, 249–259 (2003).
[12] Tan, D.-X., Manchester, L. C., Terron, M. P., Flores, L. J. & Reiter, R. J. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 42, 28–42 (2007).
[13] Reiter, R. J. et al. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim. Pol. 50, 1129–1146 (2003).
[14] Sofic, E. et al. Antioxidant capacity of the neurohormone melatonin. J. Neural Transm. 112, 349–358 (2005).
[15] Rodriguez, C. et al. Regulation of antioxidant enzymes: a significant role for melatonin. J. Pineal Res. 36, 1–9 (2004).
[16] Srinivasan, V., Spence, D. W., Pandi-Perumal, S. R., Brown, G. M. & Cardinali, D. P. Melatonin in mitochondrial dysfunction and related disorders. Int. J. Alzheimers. Dis. 2011, 326320 (2011).
[17] Tan, D.-X. et al. Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin’s primary function and evolution in eukaryotes. J. Pineal Res. 54, 127–138 (2013).
[18] Reiter, R. J. et al. Melatonin as a mitochondria-targeted antioxidant: one of evolution’s best ideas. Cell. Mol. Life Sci. 74, 3863–3881 (2017).
[19] Lowes, D. A., Webster, N. R., Murphy, M. P. & Galley, H. F. Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsi
[20] Reiter, R. J. et al. Melatonin as a mitochondria-targeted antioxidant: one of evolution’s best ideas. Cell. Mol. Life Sci. 74, 3863–3881 (2017).
[21] Reiter, R. J., Tan, D. X. & Galano, A. Melatonin: exceeding expectations. Physiology 29, 325–333 (2014).
[22] Falchi, F. et al. The new world atlas of artificial night sky brightness. Sci Adv 2, e1600377 (2016).
[23] Sliney, D. H. What is light? The visible spectrum and beyond. Eye 30, 222–229 (2016).
[24] Tosini, G., Ferguson, I. & Tsubota, K. Effects of blue light on the circadian system and eye physiology. Mol. Vis. 22, 61–72 (2016).
[25] Gooley, J. J. et al. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J. Clin. Endocrinol. Metab. 96, E463–72 (2011).
[26] Fuller, C., Lehman, E., Hicks, S. & Novick, M. B. Bedtime Use of Technology and Associated Sleep Problems in Children. Glob Pediatr Health 4, 2333794X17736972 (2017).
[27] Twenge, J. M., Krizan, Z. & Hisler, G. Decreases in self-reported sleep duration among U.S. adolescents 2009-2015 and association with new media screen time. Sleep Med. 39, 47–53 (2017).
[28] Christensen, M. A. et al. Direct Measurements of Smartphone Screen-Time: Relationships with Demographics and Sleep. PLoS One 11, e0165331 (2016).
[29] Carter, B., Rees, P., Hale, L., Bhattacharjee, D. & Paradkar, M. S. Association Between Portable Screen-Based Media Device Access or Use and Sleep Outcomes: A Systematic Review and Meta-analysis. JAMA Pediatr. 170, 1202–1208 (2016).
[30] Ohayon, M. M. & Milesi, C. Artificial Outdoor Nighttime Lights Associate with Altered Sleep Behavior in the American General Population. Sleep 39, 1311–1320 (2016).
[31] Min, J.-Y. & Min, K.-B. Outdoor Artificial Nighttime Light and Use of Hypnotic Medications in Older Adults: A Population-Based Cohort Study. J. Clin. Sleep Med. 14, 1903–1910 (2018).
[32] Everson, C. A., Laatsch, C. D. & Hogg, N. Antioxidant defense responses to sleep loss and sleep recovery. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R374–83 (2005).
[33] Tufik, S., Andersen, M. L., Bittencourt, L. R. A. & Mello, M. T. de. Paradoxical sleep deprivation: neurochemical, hormonal and behavioral alterations. Evidence from 30 years of research. An. Acad. Bras. Cienc. 81, 521–538 (2009).
[34] Venkatramanujam, S. (2011). Melatonin in Mitochondrial Dysfunction and Related Disorders. International Journal of Alzheimer’s Disease.
[35] Hardeland, R.,(2003). Oxidation of melatonin by carbonate radicals and chemiluminescence emitted during pyrrole ring cleavage. Journal of Pineal Research. 34(1):17-25.
[36] Reiter RJ, et. al. (2003). Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim Pol., 50(4):1129-46.
[37] Hardeland R,. (1993). The significance of the metabolism of the neurohormone melatonin: antioxidative protection and formation of bioactive substances. Neuroscience and Biobehavioral Reviews. 17(3):347–357.
[38] Hardeland R,. (1993). The significance of the metabolism of the neurohormone melatonin: antioxidative protection and formation of bioactive substances. Neuroscience and Biobehavioral Reviews. 17(3):347–357.
[39] Hardeland R,. (1993). The significance of the metabolism of the neurohormone melatonin: antioxidative protection and formation of bioactive substances. Neuroscience and Biobehavioral Reviews. 17(3):347–357.
[40] Leon, J, Acuña-Castroviejo, D., et. al. (2011). Melatonin and mitochondrial function. Current Topics in Medicinal Chemistry, 11: 221–240.
[41] Castroviejo DA, et. al. (2011) Melatonin-mitochondria interplay in health and disease. Current Topics in Medicinal Chemistry.
[42] Rodríguez, M.I., Escames, G., and L. C. López. (2008). Improved mitochondrial function and increased life span after chronic melatonin treatment in senescent prone ice. Experimental Gerontology. 43(8):749–756.
[43] Venkatramanujam, S. (2011). Melatonin in Mitochondrial Dysfunction and Related Disorders. International Journal of Alzheimer’s Disease.




